

Thus, in our example discussed above, oxygen, with eight protons and eight electrons, carries two electrons in its first shell and six in its second shell.

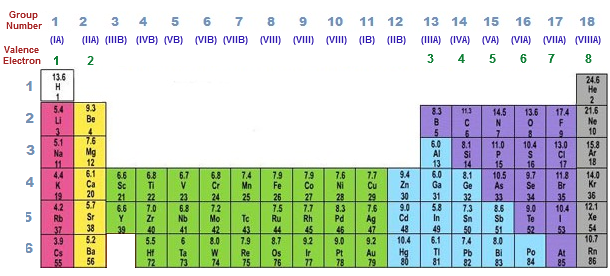
The capacity of the first electron shell is two electrons and for the second shell the capacity is eight. As lower shells are filled, additional electrons reside in more-distant shells. Each shell has a limited capacity for electrons. Located randomly around an atom's nucleus, but they occur in specific electron shells (see our Atomic Theory II module for more information). Niels Bohr's theory of the atom tells us that electrons are not In other words, the way in which an atom's electrons are arranged around its nucleus affects the properties of the atom. The "periodic" nature of chemical properties that Mendeleev had discovered is related to the electron configuration of the atoms of the elements. b.Sodium is similar to lithium in terms of chemical properties.a.Sodium comes after lithium alphabetically.Why does sodium appear directly below lithium in the periodic table? Elements in a given group in the periodic table share many similar chemical and physical properties. Columns in the periodic table are called groups. Moves from left to right in a given period, the chemical properties of the elements slowly change. Rows in the periodic table are called periods. Thus sodium begins a new row in the periodic table and is placed directly beneath lithium, highlighting their chemical Sodium (Na, z = 11), however, is a silver metal that is solid at room temperature, much like the element lithium (z = 3). The next element in order of atomic number is more similar (chemically speaking) to the first element in the row above it thus a new rowįor example, oxygen (O), fluorine (F), and neon (Ne) (z = 8, 9 and 10,respectively) all are stable nonmetals that are gases at room temperature. At the end of each row, a drastic shift occurs in chemical properties. As one moves from left to right in a row of the periodic table, the properties of the elements gradually change. The modern periodic table of elements is based on Mendeleev's observations however, instead of being organized by atomic weight, the modern table is arranged by atomic number (z). The Periodic Table of Elements Arrangement of the modern periodic table To account for these repeating trends, Mendeleev grouped the elements in a table that had both rows and columns. Mendeleev had tried to organize the chemical elements according to their atomic weights, assuming that the properties of the elements would gradually change as atomic weight increased. What he found, however, was that the chemical and physical properties of theĮlements increased gradually and then suddenly changed at distinct In 1869, the Russian chemist Dmitri Mendeleev first proposed that the chemical elements exhibited a "periodicity of properties." Understanding Scientific Journals and Articles.Using Graphs and Visual Data in Science.Scientists and the Scientific Community.


 0 kommentar(er)
0 kommentar(er)
